Court Documents for First DePuy ASR Lawsuit Reveal Manufacturer Intentionally Released Defective Device
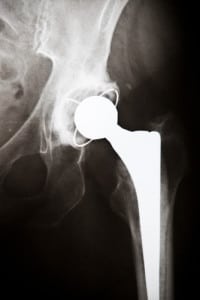
Loren Kransky’s DePuy ASR personal injury lawsuit has gone to trial, and court documents released on Friday, January 25th reveal that Johnson & Johnson knew about the all-metal hip replacements’ design flaws and high failure rates, but chose to release the defective devices to market anyway.
Even as the company began marketing versions of the all-metal hip devices in the US in 2005, they began receiving complaints from doctors and patients. In 2007, the device failed an internal test, in which its performance was compared to that of another of DePuy’s hip replacement devices. In 2011, another Johnson & Johnson internal analysis found that nearly 36% of the devices were expected to fail within 5 years.
The device was even being abandoned by surgeons who worked as consultants for the company. Still, DePuy executives continued to market the product.
DePuy executives reportedly discussed the need to fix the defect in the all-metal hip replacement devices, but never did so. According to the documents, after learning about “extreme” levels of metal ions released into patients’ blood – due to the metal ball and socket pieces grinding together – a DePuy official responded: “I believe it means that we need to start any ASR upgrade sooner than our previous plans had suggested.”
The documents released as part of Mr. Kransky’s trial reveal that Johnson & Johnson officials knew about the defect in DePuy’s ASR all-metal hip implant by 2008. The defective all-metal hip replacement devices were not recalled by Johnson & Johnson until 2010.
Johnson & Johnson Says They Acted Ethically with DePuy’s ASR Device, But Plaintiffs Claim Otherwise
By 2009, over 93,000 of the DePuy ASR hip replacement devices had been sold worldwide, with 37,000 sold specifically in the United States. Johnson & Johnson began to phase the DePuy ASR model out of production that year, claiming that sales were slowing, and the device worked as advertised. However, in mid-2010, they voluntarily recalled the ASR devices due to customer complaints.
Documents about DePuy’s and J&J’s actions were unsealed late last week as part of Loren Kransky’s personal injury case. He claims that his all-metal hip replacement failed after 5 years, despite marketing alleging that the devices should last longer than their counterparts, made from materials like plastic or ceramic.
In opening arguments, DePuy’s lawyer, Alexander G. Calfo, argued that “The evidence will show that DePuy acted as an extremely responsible manufacturer.”
A J&J spokeswoman said the company analyzed the patient registry in “looking out for patient interests” and the analysis “was based on a small, limited set of data that could not be used to generalize” the rates for replacing the hip parts. So they decided to keep the all-metal hip replacements on the market.
However, Mr. Kransky’s lawyer, Michael A. Kelly, argues differently. Regarding J&J’s tests, he said, “They did not report the data to American doctors. They changed the test and tested it against other things until they found one it could beat.”
The Strom Law Firm Can Help with Cases Against DePuy ASR and Johnson & Johnson
If you or a loved one has received a metal hip replacement device, specifically from DePuy Orthopedics, and have since suffered painful side effects, you may be entitled to compensation. The attorneys at the Strom Law Firm can help. We offer free, confidential consultations to discuss the facts of your case, so do not hesitate to contact us. 803.252.4800.

