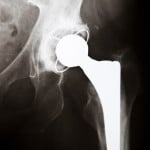
DePuy Orthopedics Will Stop Selling Two Models of Artificial Hips
Citing lack of use by doctors and surgeons, Johnson & Johnson’s DePuy Orthopedics will stop selling two types of hip replacement systems at the end of August 2013. DePuy announced on May 17th that they would discontinue both the Ultamet metal-on-metal hip replacement system, and the Complete ceramic-on-metal Acetabular systems worldwide. The decision was, according to DePuy representatives, part of a move to streamline the company’s product portfolio. “DePuy made the decision to discontinue these products because of low clinician use of Ultamet and Complete, the availability of other options that meet the clinical needs of patients and proposed changes in FDA regulation of the entire class of metal-on-metal products, which includes Ultamet,” the company said in the statement. The company said that the decision had nothing to do with recent health concerns related to two of its metal-on-metal hip replacement products, the ASR and the Pinnacle systems, and the discontinuation was not a product recall. DePuy currently faces thousands of personal injury lawsuits related to both of those products. The company said that the two products represent less than 1% of DePuy’s worldwide sales, and they would therefore discontinue the products. Surgeons currently prefer non-metal hip replacement systems, such as ceramic-on-ceramic, or ceramic-on-polyethylene. DePuy added that, as the FDA was creating a more stringent approval process for metal-on-metal hip replacement devices, they would begin to phase out models involving metal that were less popular. In part because of the health concerns related to metal-on-metal hip replacements, sales for that type of device were dropping worldwide. Ultamet is DePuy’s only current metal-on-metal hip replacement model. “We won’t have any metal-on-metal or ceramic-on metal hips any longer,” said Mindy Tinsley, a spokeswoman for J&J’s DePuy unit, said in a telephone interview. “We’ve seen, for example, a 90 percent decline in metal-on-metal sales industrywide in the U.S. andEurope since 2007. There’s really not a viable market for these bearing combinations anymore.” DePuy said it would discontinue more unpopular products in the next year, furthering efforts to simplify the company’s portfolio.
FDA Says Metal-on-Metal Hip Replacements, Like DePuy’s ASR and Pinnacle Devices, Should Be Used with Caution
According to a statement on the FDA’s website, all-metal hip implant manufacturers will have to file applications at the strictest level of government review, if the manufacturer wants to release a new all-metal hip implant device. Before FDA investigations into personal injuries began, all-metal hip implants were cleared through a less strenuous review process, called the 510(k), which has come under heavy fire lately for allowing several barely-tested, dangerous medical devices onto the market. The 510(k) process requires only that manufacturers show that their devices are substantially similar to a device that was approved previously by the FDA. Unfortunately, it will take about a year, according to agency officials, for the rules to be finalized. The new rules will give manufacturers 90 days to submit safety testing data on existing products. However, the length of time will give lobbyists the ability to oppose or modify the legislation.
The Strom Law Firm Can Help with Personal Injury Cases Related to DePuy’s All-Metal Hip Devices
If you or a loved one has received a metal hip replacement device, specifically from DePuy Orthopedics, and have since suffered painful side effects, you may be entitled to compensation. The attorneys at the Strom Law Firm can help. We offer free, confidential consultations to discuss the facts of your case, so do not hesitate to contact us. 803.252.4800.